DeepLoop robustly maps chromatin interactions from sparse allele-resolved or single-cell Hi-C data at kilobase resolution
Published in Nature Genetics, 2022
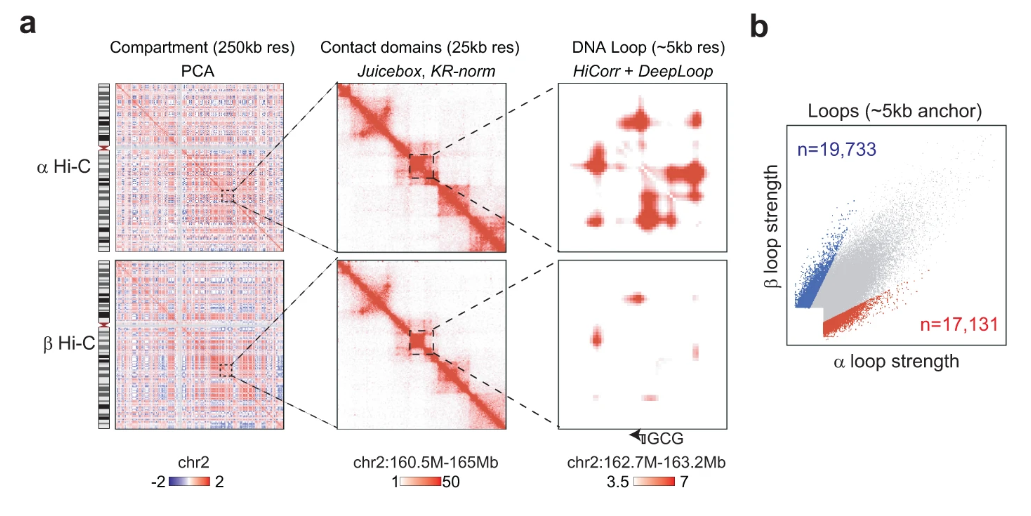
Mapping chromatin loops from noisy Hi-C heatmaps remains a major challenge. Here we present DeepLoop, which performs rigorous bias correction followed by deep-learning-based signal enhancement for robust chromatin interaction mapping from low-depth Hi-C data. DeepLoop enables loop-resolution, single-cell Hi-C analysis. It also achieves a cross-platform convergence between different Hi-C protocols and micrococcal nuclease (micro-C). DeepLoop allowed us to map the genetic and epigenetic determinants of allele-specific chromatin interactions in the human genome. We nominate new loci with allele-specific interactions governed by imprinting or allelic DNA methylation. We also discovered that, in the inactivated X chromosome (Xi), local loops at the DXZ4 ‘megadomain’ boundary escape X-inactivation but the FIRRE ‘superloop’ locus does not. Importantly, DeepLoop can pinpoint heterozygous single-nucleotide polymorphisms and large structure variants that cause allelic chromatin loops, many of which rewire enhancers with transcription consequences. Taken together, DeepLoop expands the use of Hi-C to provide loop-resolution insights into the genetics of the three-dimensional genome. 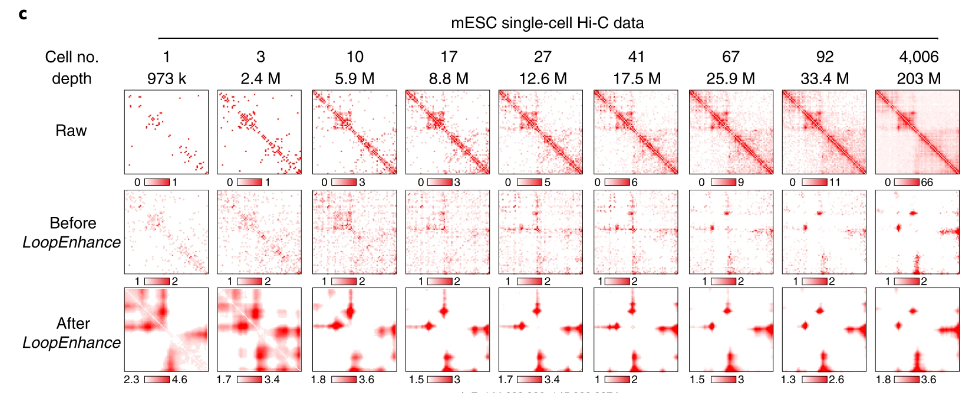
Recommended citation: Zhang, S., Plummer, D., Lu, L. et al. DeepLoop robustly maps chromatin interactions from sparse allele-resolved or single-cell Hi-C data at kilobase resolution. Nat Genet 54, 1013–1025 (2022). https://doi.org/10.1038/s41588-022-01116-w https://www.nature.com/articles/s41588-022-01116-w